Seeking a Better Understanding of Atmospheric Mercury
The Air Resources Laboratory measures and models atmospheric mercury to provide essential information to policy-makers and planners
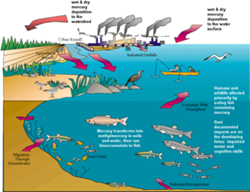
It may be the name of a car, a planet, and a Roman god, but (as best we know) none of those are toxic to humans or the environment. Mercury, a natural element of the Earth, is now better known as a potentially deadly metal. It is a potent neurotoxin, particularly damaging to the development of fetuses, infants and young children. Our exposure to methylated mercury, the most toxic form, is largely through eating contaminated fish.
Today, nearly every U.S. state warns residents to restrict their consumption of certain fish due to mercury contamination. The U.S. Food and Drug Administration and U.S. Environmental Protection Agency also have fish consumption advisories for mercury. In addition, new research indicates that mercury also is accumulating at potentially dangerous levels in terrestrial wildlife, some of which are not fish-eating animals.
But mercury doesn't start out in living organisms, it gets there. Through mostly human activities (and some natural sources too), mercury is released from ores, minerals and fossil fuels into the biosphere where it can circulate throughout the globe and accumulate in living organisms.
Although many uses of mercury have been curtailed, mercury compounds continue to be released into the atmosphere. The largest sources of mercury emissions in the U.S. and worldwide are coal-fired power plants, waste incinerators, metallurgy/mining operations (especially gold mining), and chlor-alkali plants that employ mercury-cell technology. A potentially growing source of mercury is disposable products, such as compact fluorescent bulbs and personal electronics (cell phones, LCD TVs, digital cameras), which contain mercury. The mercury eventually leaks into the atmosphere when broken or crushed in a landfill.
What is tricky about mercury is that it exists in a variety of chemical and physical forms and can change from one form to another. Some forms are more toxic than others. Depending on its form, mercury released into the air can travel short or long distances even all the way around the world - and cycle between the earth's surface and the atmosphere. Unless it is contained, mercury can be re-emitted into the air.
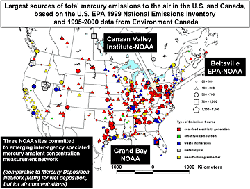
Concentrations of mercury in the air are usually low and are generally not an air quality concern. It is when mercury deposits to land and water surfaces that it becomes an issue. While measuring mercury in precipitation is relatively easy, measuring ambient air concentrations and understanding how and where it deposits in dry form is quite another story. These challenges make it difficult to understand the relative contribution of mercury to ecosystems from various geographic regions and types of sources. Such understanding is essential for developing effective regulations and policies.
Scientists at NOAA's Air Resources Laboratory (ARL) have taken on this challenge. Teaming up with U.S. EPA and other scientists, ARL researchers are using their measurement capabilities at three core mercury monitoring sites: Beltsville, Maryland; Grand Bay Mississippi; and Canaan Valley, West Virginia. These sites are part of a new multi-agency national monitoring network designed to address total mercury deposition across the country.


Each site monitors important mercury species (elemental, reactive gaseous, and particulate) in the air using sophisticated mercury analyzers. Some sites also measure mercury in precipitation and snow pack. These data are then correlated with other air quality data and meteorological measurements to provide an overall sense of how the airborne mercury is transported and deposited.
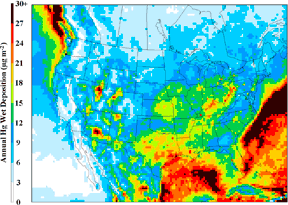
Yet, monitoring and measurement studies alone do not conclusively reveal the origin of the mercury or how it is transformed as it mixes with other pollutants in the air. To address these and other issues, ARL researchers turn to their mercury modeling capabilities. Using state-of-the-art atmospheric fate and transport models (CMAQ and HYSPLIT), scientists are attempting to track mercury in the atmosphere from emissions sources to eventual deposition. These models make use of detailed NOAA weather data and emissions inventories from U.S. EPA and other agencies. The mercury models are then interpreted and evaluated using measurements of ambient air concentrations and deposition, including measurements collected at NOAA's core sites.
Through the integration of monitoring and modeling tools, ARL scientists are able to provide the Nation with the capability to identify important sources contributing mercury to ecosystems and to estimate the consequences of potential alternative policies for reducing mercury emissions. Air quality policymakers and planners require that information to both effectively protect public health and maintain a vibrant economy.
 Deep Sea Crabs
Deep Sea Crabs